
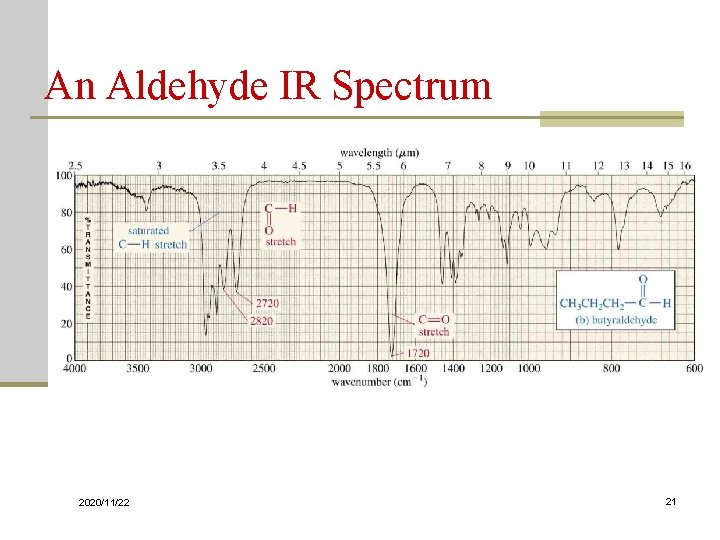
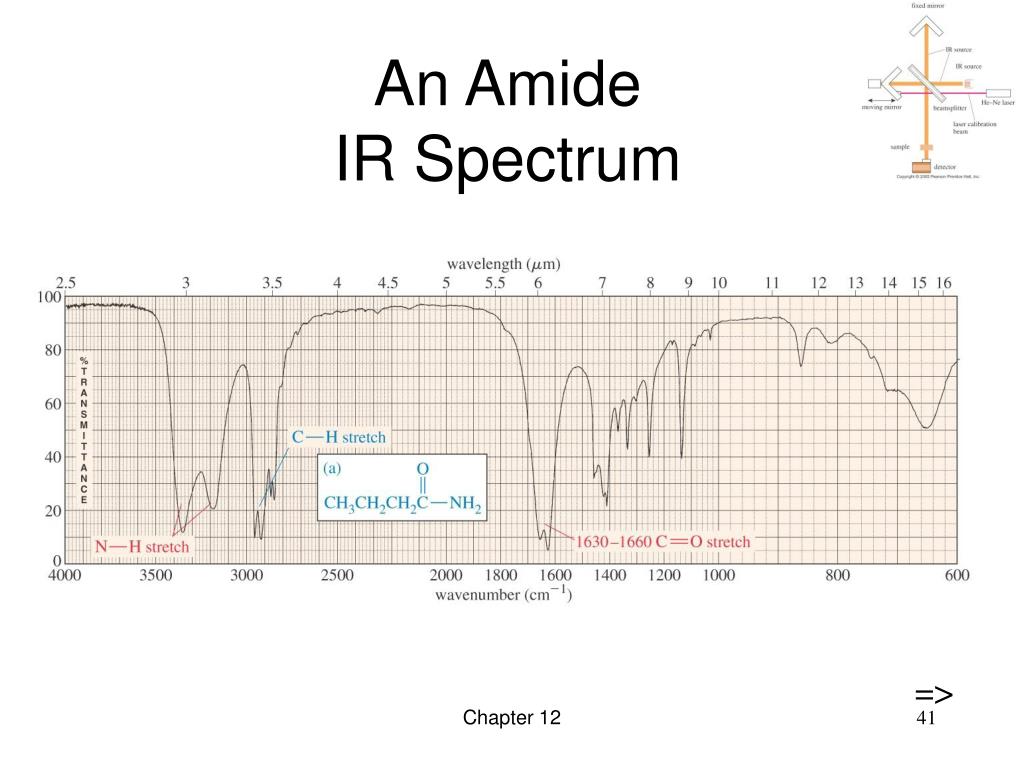
The larger wavenumbers (shorter wavelengths) are associated with higher frequencies and higher energy. Please note that the direction of the horizontal axis (wavenumber) in IR spectra decreases from left to right. The wavenumber is defined as the reciprocal of wavelength ( Formula 6.3), and the wavenumbers of IR radiation are normally in the range of 4000 cm-1 to 600 cm-1 (approximate corresponds the wavelength range of 2.5 μm to 17 μm of IR radiation).
The vertical axis is ‘% transmittance’, which indicates how strongly light was absorbed at each frequency.
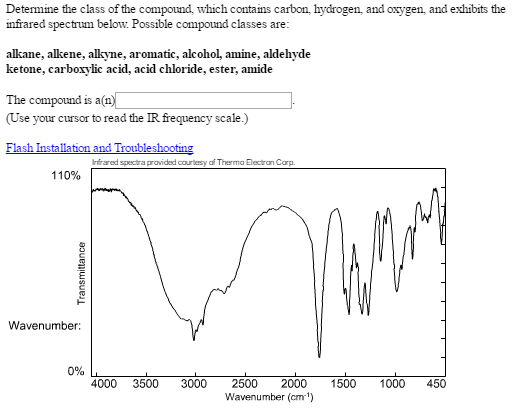
Aldehyde ir spectrum how to#
With a basic understanding of IR theory, we will now take a look at the actual output from IR spectroscopy experiments and learn how to get structural information from the IR spectrum. 6.3 IR Spectrum and Characteristic Absorption Bands


 0 kommentar(er)
0 kommentar(er)
